A guide on reporting potential medical supply disruptions has been published by NHS England.
The guidance ‘Reporting potential supply disruptions of medical equipment and consumables’ aimed at ICBs and others sets out a step-by-step process for what to do when an issue is identified.
There are six steps in the guidance. The first of which asks for initial views on product use and the acceptability of alternatives to be sought if a trust/ICB or clinical network realises there is an actual or potential disruption to the supply of medical equipment and consumables – for example, a company notifies them of this.
The guidance says that clinical and procurement professionals’ views are essential. If a trust has identified a potential problem, it should liaise with the ICB heads of procurement where these posts exist.
ICBs without a head of procurement post should establish and communicate the official/team that is the designated point of contact for providers and NHSE in these circumstances.
The guidance has set out a checklist to ensure the correct information is captured (see box).
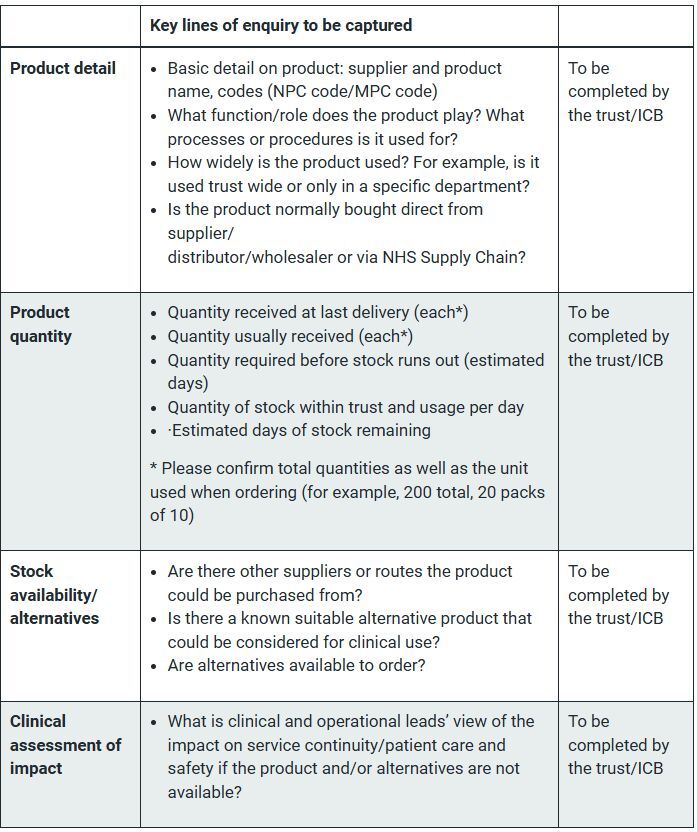
Once ICBs have ascertained that there is an issue, step two is to discuss potential mitigations with NHS Supply Chain and/or the supplier.
If the product is normally ordered via NHS Supply Chain, ICBs should log in to the NHS Supply Chain’s website to check why the item is unavailable and whether there are details on alternatives.
They should also contact their NHS Supply Chain contact for support in managing the disruption, such as whether residual stock can be found or suggested alternatives to the item.
They should also discuss with clinical colleagues whether any available direct or indirect alternatives could be considered an interim substitution.
Where the product is not purchased via NHS Supply Chain, ICBs should follow the same process with existing suppliers.
If step two does not resolve the issue, step three is for the ICB to support trusts to liaise with other organisations in the ICB, the ICB emergency preparedness, resilience and response (EPRR) lead and, where relevant, system partners such as social care to understand stock levels and if peer organisations are facing the same challenges.
The guidance says that discussions should take place about whether mutual aid is available within the ICB on a temporary or long-term basis.
If mutual aid is not available within the ICB or offers only a temporary solution to a long-running disruption, then trusts/ICBs should move to step four and raise it with the National Supply Disruption Response (NSDR).
In step five, NSDR investigates.
Although a trust/ICB, manufacturer, supplier, NHS Supply Chain, DHSC workstream, or any health or care organisation can report a disruption, the NSDR will only inform the ‘originator’—the organisation that reports the problem—of the case investigation. The frequency of updates will be determined on a case-by-case basis.
The guidance states that the trust/ICB or clinical network should not repeatedly raise disruptions once NSDR has opened a case.
At the same time as the investigation, ICBs should use the information gathered using the checklist in step one to provide a briefing to support formal escalation to regional operation centres (ROCs).
At this point, ICBs are requesting formal support from regional EPRR to manage the disruption, such as seeking inter-regional mutual aid.
The final step is an escalation to national EPRR, which is a decision taken by regional EPRR teams or ROCs.
The guidance adds that medicine shortages and/or disruptions are out of scope of this process. They will be managed by the NHSE Commercial Medicines Unit and/or the Department of Health and Social Care medicines team.






