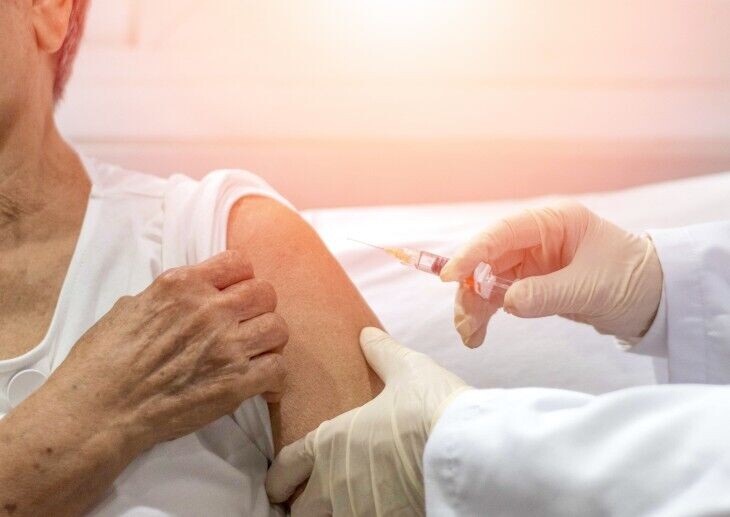
A new ‘super-jab’ for 15 types of cancer expected to save 1,000 hours of treatment time every month will be rolled out from May, announced NHS England.
The injectable form of immunotherapy, nivolumab – one of the most widely used cancer treatments – means patients can receive their fortnightly or monthly treatment in five minutes instead of up to an hour via an IV drip.
The super-jab could save around 1,000 hours of treatment time every month – the equivalent of more than one full year of time annually.
As well as freeing staff to deliver more appointments and treatments, it will help address demand in cancer day units where the drug is currently administered.
The new jab can be used to treat 15 cancer types, including skin cancer, bladder, and oesophagus.
It is estimated around two in five patients who currently receive IV nivolumab should be eligible for the super-jab – about 1,200 patients in England per month.
The faster treatment comes at no extra cost to the NHS because of an agreement negotiated by NHS England with the manufacturer Bristol Myers Squibb.
Elizabeth O’Mahony, NHS England chief financial officer said the super-jab was ‘a huge boost for people going through cancer care, helping them to spend less time in hospital’.
‘It’s also a major win for the NHS, saving the equivalent of a year’s worth of treatment time which can be used to deliver other care, building on the great strides made in the past six months, and thanks to a deal struck by NHS England this quick treatment will be available without any additional cost,’ she said.
James Richardson, clinical pharmacist and National Specialty Adviser for Cancer Drugs said he was ‘delighted’ about the roll-out.
‘This is a significant advancement in cancer treatment, with the potential to improve the lives of thousands of patients each month,’ he said.
The announcement of the roll-out follows approval from the Medicines and Healthcare Products Regulatory Agency (MHRA) at the end of April.
In clinical trials, patients were highly satisfied with the under-the-skin injection, which takes 3-5 minutes to administer, and preferred it to the IV form of the drug which takes 30 to 60 minutes every two or four weeks, depending on the cancer type.
NHS cancer services will now be preparing to treat the first patients with the new super-jab treatment this month when supplies of the product are received in the UK.